A Review of Rick Bright’s Leaked Emails from Early 2020
Rick Bright was instrumental in ensuring hydroxychloroquine was denied to Americans in need. We found and reviewed his leaked emails. Some of these emails were featured in "Epidemic of Fraud".
In the spring of 2020, there was a flurry of confusion about hydroxychloroquine and the associated risks of taking one of the oldest, safest classes of medications in human history. Rick Bright was the head of BARDA, an HHS agency known as the ‘venture capital’ wing of the HHS. Rick was directly responsible for crafting the Emergency Use Authorization, in tandem with Janet Woodcock and others at the FDA, that limited the drug’s availability in the period of time it would be most effective – before and immediately after exposure to COVID. When Rick Bright was dismissed from his role at BARDA for leaking emails to the press to embarrass HHS leadership he wanted to ‘rein in’, he attempted to claim whistleblower status and presented limited, cherry-picked emails to a House subcommittee headed by Democrat congresswoman Anna Eshoo, who at the time was one of the largest recipients of pharma cash in the House of Representatives.
These are Rick Bright’s leaked emails.
Broken Truth has submitted FOIA requests for all emails between Rick Bright and Janet Woodcock at the FDA. We have been told it will take the FDA 24 months to release those communications.
We have reviewed the emails Rick Bright leaked to the news media when he attempted to ‘rein in’ his leadership from HHS in the middle of a pandemic. The results show a greedy agency more interested in dangerous, expensive drugs like Remdesivir over proven drugs like Hydroxychloroquine. For some strange reason Bright gave an incredible amount of weight to the opinions of a mask manufacturer over his own leadership at HHS.
Bright’s leaked emails reveal an agency that was ignorant to basic treatment regimens and was predominantly staffed by PhD.’s instead of medical doctors.
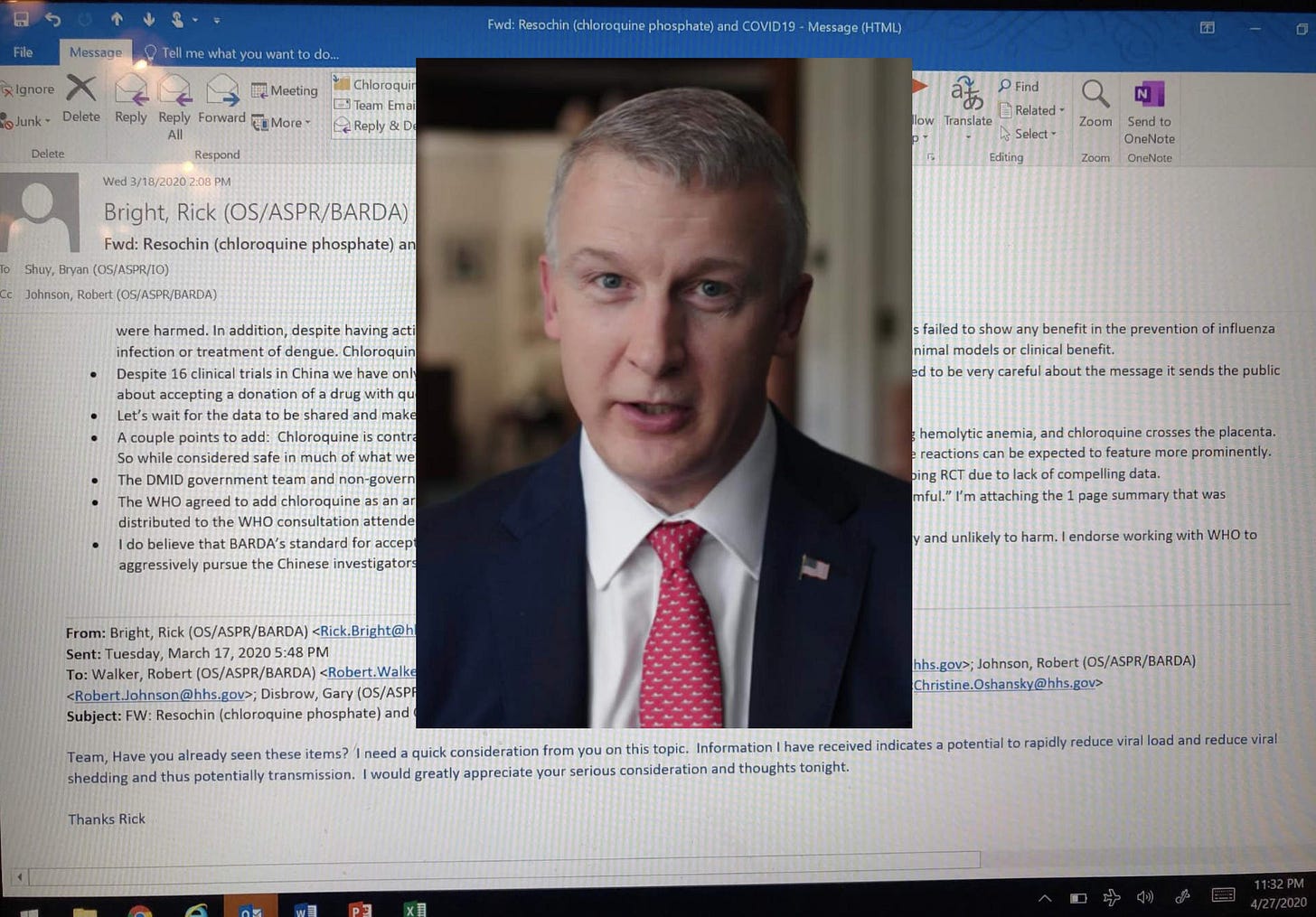
Rick Bright and Gary Disbrow weren’t going to help with COVID without funding.
If you called 911 and the firefighters told you they wanted new fire trucks before they would help put your fire out, especially after you gave them over a billion dollars a year to put fires out, how would you respond? As early at January 13, 2020 BARDA was already digging heels in about getting greased with taxpayer cash. BARDA had a 1.6 billion dollar budget in 2020.
BARDA knew about Chloroquine as a potential treatment option in Feb of 2020 and DID NOTHING
In this March 17 email from Bright to his staff at BARDA, he asked about chloroquine and BARDA came up with rejections of the drug almost immediately. Interestingly, they admitted knowing about the Nature In Vitro study from Feb 4, 2020 authored by the Wuhan Institute of Virology and the notorious ‘bat lady’ from Wuhan. It’s very curious that the WIV was even interested in looking at remdesivir in this study. The Chinese never recommended treatment with Remdesivir, but the Chinese DO recommend chloroquine.
Christopher Houchen’s response on behalf of the “Team” at BARDA? Desperate attempts to discredit HCQ and not accept the massive donation to the Strategic National Stockpile that could have ended the pandemic quickly.
Of note, BARDA’s desperate attempts to discredit HCQ even went so far as to claim G6PD deficiency was an issue. However, studies showed that at the doses needed to protect that G6PD was not an issue with HCQ. What’s worse though, is that G6PD IS an issue with Remdesivir, yet the WHO and FDA had no concern with that despite organ failure being a significant issue with Remdesivir.
Bright was on board the Remdesivir Train in Feb 2020
Despite the cell research / nature article showing affordable HCQ was more beneficial than the very deadly remdesivir, he was already trying to source Remdesivir on Feb 10, 2020. This is not the behavior of an official eager to find a ‘quick fix’ to a pandemic.
In Senate testimony, Dr. Paul Marik testified about how his hospital demanded he use remdesivir and restricted use of basic steroids like methyl-prednesilone, much like Teladoc is allegedly doing now.
In a surprise to no one, studies indicate that methylprednisolone is more effective against COVID-19 pneumonia than other steroids like dexamethasone. So why on earth are hospitals denying access to it and firing doctors like Dr. Marik?
We have asked Teladoc for comment on it’s policy to fire physicians who prescribe steroids like methylprednisolone. Perhaps we will hear back.
BARDA’s budget in 2020 Tripled thanks to COVID
Good thing Disbrow and Bright like money because they got it in spades in 2020. According to StatNews:
Lawmakers were so confident that BARDA could help scientists develop a coronavirus vaccine, therapy, or even a diagnostic test that Congress has showered the agency with a $3.5 billion boost in funding, more than tripling its total budget.
In late 2019 Bright and Fauci discussed ‘blowing the system up’ and creating an entity of excitement to change the game in flu vaccines. Is this what happened in 2020? See an edited down version of the presentation as featured in “Epidemic of Fraud.
Epidemic of Fraud (Remastered audio)
Epidemic of Fraud is the award-winning documentary that explores the bizarre media, medical, and partisan political attacks levied against a class of ancient medications, told from the perspective of a former CNN journalist and Hollywood industry veteran. Why were the people who allowed the fentanyl disaster to go unchecked so eager to discredit a drug …
Rick Bright PhD left the government in 2020. On Nov 9, 2020, President-elect Joe Biden named Bright to be one of the 13 members of his coronavirus task force.
In March 2021 Bright was hired by the Rockefeller Foundation as Senior Vice President of Pandemic Prevention and Response. The foundation is part of the World Economic Forum.
Many thanks to Marion Meiners for contributing to this report.
You can download all Rick Bright’s emails below.






Relatedly— Dr. Harvey Risch Calls Out the FDA's HCQ Fraud
Source video:
Senator Ron Johnson's Roundtable, COVID-19: A Second Opinion
Streamed on January 24, 2022
https://rumble.com/vt62y6-covid-19-a-second-opinion.html
TRANSCRIPT
1:00:44
SENATOR RON JOHNSON: Our next presenter is Dr. Harvey Risch. Dr. Risch is a professor of epidemiology at Yale School of Public Health.[1] He has been a university epidemiologist for more than 40 years and is a fellow of the American College of Epidemiology and a member of the Connecticut Academy of Science and Engineering. After getting his MD degree he completed a PhD in mathematical modeling of infectious epidemics. He has published more than 400 scientific research papers that have been cited more than 44,000 times.
I just, quick little aside here, Dr. Risch and Dr. McCullough joined me with Dr. George Fareed who can't be with us today, in November of 2020, in my first hearing on early treatment.[2] Following that, the New York Times published a article, a column written by the Democrat witness of that hearing, Dr. Ashish Jha, who had never treated a covid patient, I actually read an article later, he holed up in his apartment for like over a year til he got a vaccine. But the New York Times titled that paper, or that column, "The Snake-Oil Salesmen of the Senate."[3]
I want people to know that because, as you listen to Dr. McCullough, as you listen to Dr. Risch, ask yourself, do they really seem to be snake-oil salesmen to you? They seem to be eminently qualified professionals, that again, in Dr. McCullough's case, has had the courage and compassion to actually treat covid patients. Dr. Risch.
1:02:21
DR. HARVEY RISCH: Thank you, Senator, colleagues, listeners. It's my honor to be addressing you today and to answer questions later.
We heard at the beginning of the pandemic that one of the medications that has been used in early treatment, hydroxychloroquine or HCQ, was a game changer and would be effective in the treatment of covid outpatients starting during the first few days of the illness. And then we heard study after study, and media report after media report, saying that HCQ doesn't work. These negative claims continued for months until the media got bored with all this and then acted as if the case were closed.
However, this was a sham.
The media reports never covered how the negative studies were actually fake studies. While they did cover the Surgisphere fraud[4], both the study that was published that was retracted but that managed to change the WHO's policy before it got retracted, and the media never covered how the randomized trials that were put out that were supposedly informative about the lack of benefit of hydroxychloroquine had hid their positive results, were designed for low-risk people who never had any real risk for hospitalization or death outcomes, were not blinded, or had no idea who their Internet participants really were, or any of the other numerous flaws that made them essentially irrelevant.
And the media studiously avoided covering the 10 proper trials of hydroxychloroquine outpatient use that showed significant benefit for hospitalization and mortality.
[turns to chart on display]
And just as a quick aside, the top two figures are for hydroxychloroquine for hospitalization risk and mortality risk. To the left of the vertical line means benefit. The diamond means how big the error, the range of possible values are. There's very significant 50% reduced risk for hospitalization, 75% reduced risk of mortality. And just for comparison, you can see very similar results for ivermectin in the bottom two trials. OK. This is real evidence. This is real scientific evidence.
Now the media has not reported any of these studies, but that does not make them nonexistent. These studies involve, the hydroxychloroquine studies involve more than 40,000 patients including nation-wide studies in two countries. So we see here that early hydroxychloroquine use dramatically reduces the risk of hospitalization and mortality. Now we could later, or never if you want, discuss randomized versus nonrandomized trials, the scientific issues involved in that, but what you've seen here is essentially scientific proof.
Given that, why aren't doctors across the US actually prescribing hydroxychloroquine as part of early outpatient treatment? Well, in fact, early in 2020 doctors did start using hydroxychloroquine in outpatients. But this was short-circuited by an act of FDA and BARDA[5] employees to use the Emergency Use Authorization regulations to block hydroxychloroquine use in outpatients except in randomized trials. And these trials that are the same ones that would be cut off by participant fear because of the Surgiphere papers.
And then the FDA mounted its biggest fraud of all times—
[Dr. Risch stands up, turns around, brings forward another large chart, then resumes his seat]
—by putting up this warning. This warning says, FDA cautions against the use of hydroxychloroquine in outpatients outside of the hospital setting. But then, in the justification, it says, "We base this on information to treat hospitalized patients."
Hospital disease, as we'll hear, and as we know from 2 years of dealing with covid, is a completely different illness treated with different drugs, different medications, in the hospital. Outpatient disease is flu-like, hospital disease is a florid pneumonia. And so the fact that the FDA would base recommendations and warnings on hospital disease, which is a totally different disease than outpatient disease, is a fraud.
This website is still there today and constitutes an outright fraud. OK. This basically scared everyone across the country against using this, on the basis of this fraudulent website.
Now Senator Johnson has twice demanded from the FDA, by in writing, to release the data that they relied upon to make this claim that, of warning, and twice the FDA refused.
So at this point, we know it works, we have lots of medicines, not just hydroxychloroquine, not just ivermectin for that matter, that need to be used. And the FDA has to be held accountable for this website.
Thank you.
1:07:23
[END]
# # #
TRANSCRIBER'S NOTES:
[1] Dr. Harvey Risch is Professor Emeritus and Senior Research Scientist in Epidemiology (Chronic Diseases); Affiliated Faculty, Yale Institute for Global Health
https://ysph.yale.edu/profile/harvey-risch/
Twitter: https://twitter.com/DrHarveyRisch
Telegram: https://t.me/HarveyRischMDPhD
See also archived posts to-date by Dr. Risch on the Brownstone Institute site:
https://brownstone.org/author/harvey-rische/
See also Totality of Evidence archived Dr. Harvey Risch links:
https://totalityofevidence.com/dr-harvey-risch/
[2] See: Early Outpatient Treatment: An Essential Part of a COVID-19 Solution
November 19, 2020
https://www.hsgac.senate.gov/hearings/early-outpatient-treatment-an-essential-part-of-a-covid-19-solution/
The webpage includes the panelists' statements, including that of Dr. Harvey Risch:
https://www.hsgac.senate.gov/wp-content/uploads/imo/media/doc/Testimony-Risch-2020-11-19.pdf
See also the statement of Dr. George Fareed:
https://www.hsgac.senate.gov/wp-content/uploads/imo/media/doc/Testimony-Fareed-2020-11-19.pdf
[3] The Snake-Oil Salesmen of the Senate
There is no evidence that hydroxychloroquine helps Covid/.19 patients. So why is Congress still holding hearings on it?
By Dr. Ashish Ja, November 24, 2020
https://www.nytimes.com/2020/11/24/opinion/hydroxychloroquine-covid.html
[4] See: "RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis"
Mehra et al, The Lancet, May 22, 2020
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2820%2931180-6/fulltext
See also:
"A mysterious company's coronavirus papers in top medical journals may be unraveling
Scientists and journals express concern over influential studies of COVID-19 patient data that evaluated possible treatments such as hydroxychloroquine"
2 Jun 2020
By Kelly Servick, Martin Enserink
https://www.science.org/content/article/mysterious-company-s-coronavirus-papers-top-medical-journals-may-be-unraveling
And see:
"The Surgisphere Scandal: What Went Wrong?
The high-profile retractions of two COVID-19 studies stunned the scientific community earlier this year and prompted calls for reviews of how science is conducted, published, and acted upon. The warning signs had been there all along."
Catherine Offord, October 1, 2020
https://www.the-scientist.com/the-surgisphere-scandal-what-went-wrong--67955
[5] FDA is the U.S. Food and Drug Administration. https://www.fda.gov/
And BARDA is the U.S. Biomedical Advanced Research and Development Authority
https://www.hhs.gov/about/agencies/orgchart/aspr/barda/index.html